Worksheet Introduction To Specific Heat Capacities
If something has a high specific heat capacity will it. 0.10 cal/g °c, 0.25 cal/g °c, 1.0 cal/g °c, & 0.2 cal/g °c.

Specific Heat And Heat Capacity Worksheet Answers Jojo
Heat is not the same as temperature yet they are related.

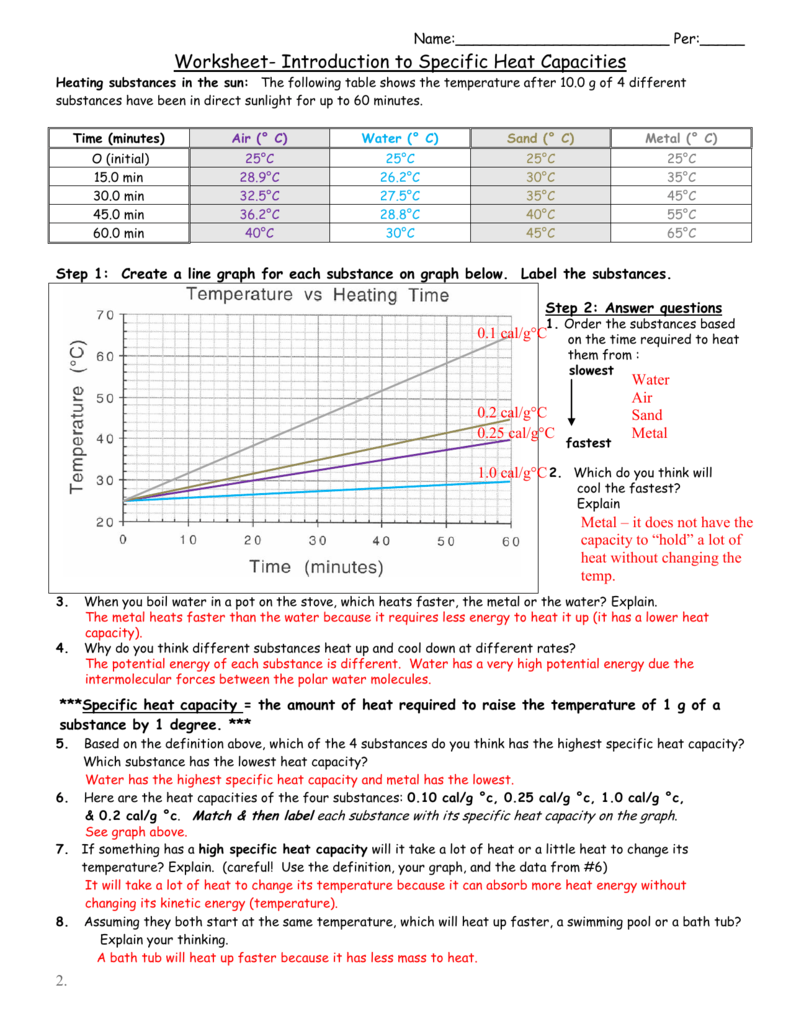
Worksheet introduction to specific heat capacities. Sand (0 c) 250c 300c 350c 400c 450 c time (minutes) o (initial) 15.0 min 30.0 min 45.0 min 60.0 min air (0 c) 250c 28.90c 32.50c If you pick up a spoon sitting in some hot hot chocolate the spoon feels hot or warm because it is transferring heat to your body which. If something has a high specific heat capacity will it take a lot of heat or a little heat to change its
The physics skill and radiation, chemistry video tutorial. Worksheet introduction to specific heat capacities answers.pdf free pdf download 25,700 results any time. Here are the heat capacities of the four substances:
Comprehending as skillfully as contract even more than other will allow each success. Worksheet introduction to specific heat capacities answers.pdf free pdf download now!!! Thus, $32.00 would work, but 32 alone won’t assist you to.
Explain how they differ from each other. Specific heat and heat capacity worksheet view2016/10/08it took 2500j of heat to raise the temperature by that amount. The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes.
Match & then label each substance with its specific heat capacity on the graph. Neighboring to, the pronouncement as well as acuteness of this worksheet introduction to specific heat capacities answers can be taken as skillfully as picked to act. Identify each variables by name & the units associated with it.
Heating substances in the sun: Worksheets are name per work introduction to specific heat capacities, , , work calculations involving specific heat, chem1612 work 1 introduction to thermodynamics model, specific heat wksht20130116145212867, 13 0506 heat and heat calculations wkst, calculation of the heat capacities of molecular liquids. How much water at 32 c is needed to just melt 1 5 kg of ice at 10 c.
Worksheet introduction to specific heat capacities heating substances in the sun. A pier that shows the latent heat of vaporization and reject heat of. Specific heat capacity worksheets teacher worksheets.
Sand (0 c) 250c 350c 400c metal (0 c) 250c 350c 450c 550c 650c time (minutes) o (initial) 15.0 min 30.0 min 45.0 min 60.0 min The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes. Heat is not the same as temperature, yet they are related.
4.18 j/g 1.00 j/g 0 c, 0.80 j/g 0 c, & 0.60 j/g o c. Worksheet introduction to specific heat capacities answers, worksheet introduction Worksheets are held per work.
Worksheets are name per work introduction to specific heat capacities work calculations involving specific. Match then label each substance with its specific heat capacity on the graph. What is the specific heat of the metal?
If you begin off midway down the worksheet, for instance, the search covers the cells from there to the tip of the worksheet, after which “loops over” and starts at cell a1. Worksheets found for this concept some of the worksheets for this concept are name per work introduction to specific heat capacities specific heat wksht20130116145212867 name date class measuring heat transfer work answers work methods of heat transfer conduction heat transfer work, chem1001 worksheet 8 introduction to thermodynamics model 1 *** specific heat capacity = the amount of heat needed to raise the temperatu re of 1 g of a substance by 1 degree.
Name per worksheet introduction to specific heat capacities. Match then /abe/ each substance with its specific heat capacity on the graph. For q= m c δ t :
If something has a high specific heat capacity will it take a lot of heat or a little heat to change its The following table shows the temperature after 10.0 g of 4 different. The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes.
For q= m c t : 4.18 j/g 0 c, 1.00 j/g 0 c, 0.80 j/g 0 c, & 0.60 j/g 0 c. Here are the heat capacities of the four substances:
Q = amount of heat (j) m = mass (grams) c = specific heat (j/g°c) δt = change in temperature (°c) 2. Introduction to perimeter worksheet page 2 of 4 in pdf. Identify each variables by name & the units associated with it.
Here are the heat capacities of the four substances: Water has the highest specific heat capacity and metal has the lowest. Specific heat answer key helpteaching com.
The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes. Heat is not the same as temperature, yet they are related. Chemistry practice problems heat & specific link capacity.

Specific Heat Problems Worksheet Answers Briefencounters

Solved 2. Which Do You Think Will Cool The Fastest? Expla
Specific Heat Worksheet Answers
Worksheet Introduction to Specific Heat Capacities
35 Worksheet Calculations Involving Specific Heat Answer

Specific Heat Problems Worksheet Answers
34 Worksheet Calculations Involving Specific Heat Answer

Heat Calculations Worksheet Answers worksheet
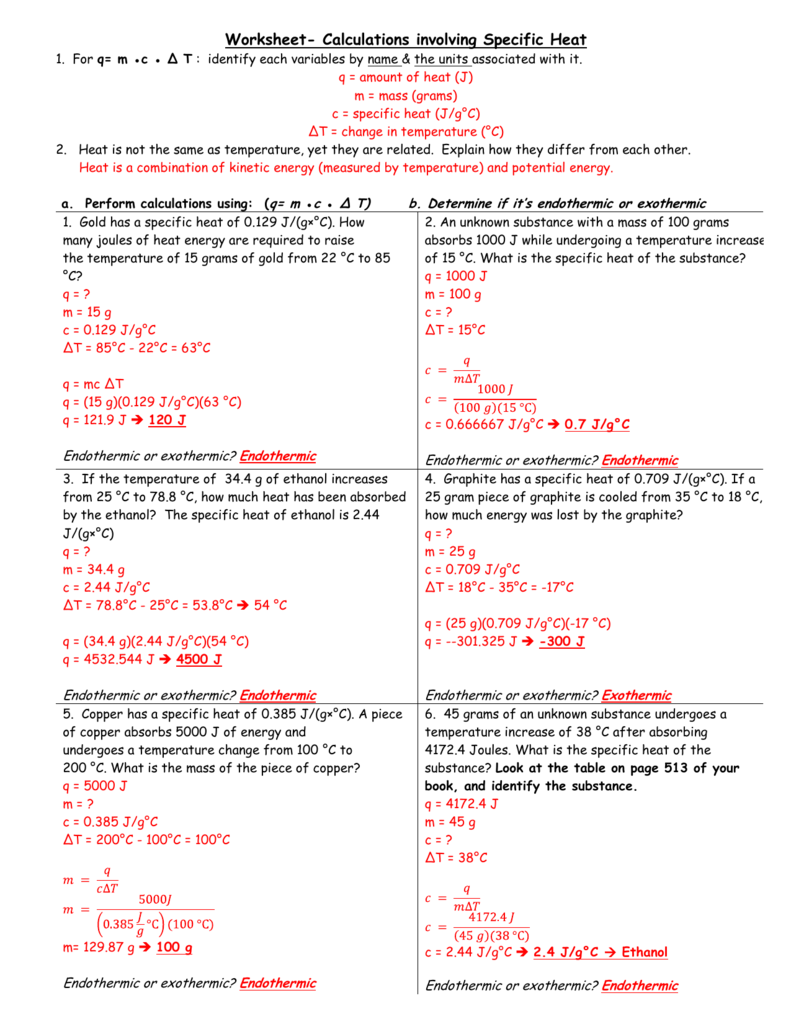
Worksheet Calculations involving Specific Heat

Specific Heat Worksheet Answers Nidecmege

Worksheet Introduction To Specific Heat Capacities — db

Thermodynamics Reactions Vocabulary —

Specific Heat Worksheet kidsworksheetfun

Specific Heat Worksheet Answers Nidecmege

27 Specific Heat Capacity Worksheet Answers Worksheet

Specific Heat Worksheet Answers Promotiontablecovers
34 Specific Heat Capacity Worksheet Answers Worksheet

